Answer:
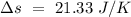
Step-by-step explanation:
Given,
- Mass of the ice =
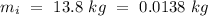
- Temperature of the ice =
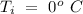
- Mass of the original water =
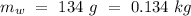
- Temperature of the original water =
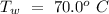
- Specific heat of water =
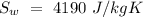
- Latent heat of fusion of ice =
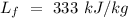
Let T be the final temperature of the mixture,
Therefore From the law of mixing, heat loss by the water is equal to the heat gained by the ice,
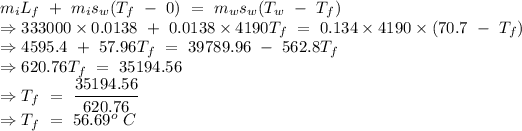
Now, We know that,
Change in the entropy,
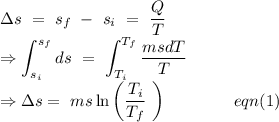
Now, change in entropy for the ice at 0^o\ C to convert into 0^o\ C water.
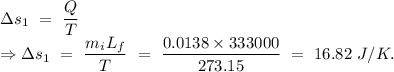
Change in entropy of the water converted from ice from
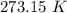 to water 330.11 K water.
to water 330.11 K water.
From the equation (1),
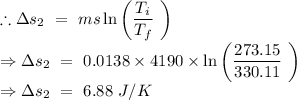
Change in entropy of the original water from the temperature 342.85 K to 330.11 K
From the equation (1),
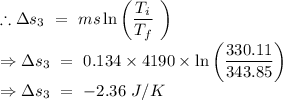
Total entropy change =
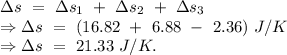
Hence, the change in entropy of the system form then untill the system reaches the final temperature is 21.33 J/K