Answer:
131.4 mg/L of oxygen is needed to biodegrade the organic compound.
Step-by-step explanation:
The chemical reaction will be written as:
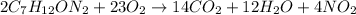
Concentration of the organic compound = 50 mg/L
This means that 50 milligrams of organic compound in present in 1 L of the solution.
50 mg = 0.050 g
1 mg = 0.001 g
Moles of organic compound =
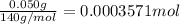
According to reaction, 2 moles of organic compound reacts with 23 moles of oxygen gas.
Then 0.0003571 moles of an organic compound will react with:
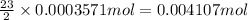 oxygen gas.
oxygen gas.
Mass of 0.004107 moles of oxygen gas:
0.004107 mol × 32 g/mol = 0.1314 g = 131.4 mg
131.4 mg/L of oxygen is needed to biodegrade the organic compound.