Answer:
W=-940.36 KJ
Step-by-step explanation:
Given that
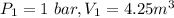
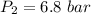
Process follows pv=constant
So this is the isothermal process and work in isothermal process given as
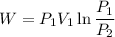
Now by putting the values (1.4 bar =140 KPa)
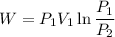
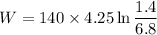
W=-940.36 KJ
Negative sign indicates that this is a compression process and work will given to the system.