Step-by-step explanation:
The given data is as follows.
Mass of hexane = 10 kg, Mass of octane = 30 kg
Formula to calculate average density of mixture is as follows.
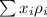
where,
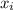 = mass fraction of i component in mixture
= mass fraction of i component in mixture
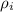 = density of i component
= density of i component
Hence, calculating the mass fraction of both hexane and octane as follows.
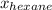 =
=
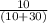 = 0.25
= 0.25
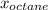 =
=
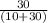 = 0.75
= 0.75
Therefore, calculate the average density as follows.
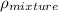 =
=
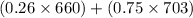
= 692.25
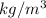
Thus, we can conclude that the average density of given hexane/octane mixture is 692.25
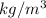 .
.