Answer:
1160 K.
Step-by-step explanation:
Given that
Initial
Pressure P =600 KPa
Temperature T =290 K
Volume V =0.01
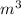
If we assume that air is s ideal gas the
P V = mRT
R=0.287 KJ/kg.k
now by putting the values in above equation
600 x 0.01 = m x 0.287 x 290
m=0.07 kg
The work out at constant pressure given as
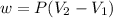
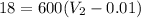
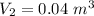
At constant pressure
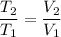
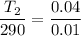
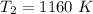
So the final temperature is 1160 K.