Step-by-step explanation:
The given data is as follows.
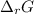 = -16.7 kJ/mol =
= -16.7 kJ/mol =
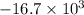 , T = 298 K
, T = 298 K
R = 8.314 J/mol K,
 = ?
= ?
Relation between
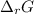 and
and
 is as follows.
is as follows.
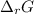 =
=
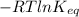
Hence, putting the values into the above equation as follows.
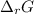 =
=
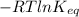
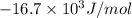 =
=
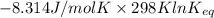
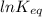 =
=
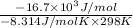
= 6.740
 = antilog (6.740)
= antilog (6.740)
= 846
Thus, we can conclude that
 for given values is 846.
for given values is 846.