Answer:
0.4429 m
Step-by-step explanation:
Given that mass % of the ethanol in water = 2%
This means that 2 g of ethanol present in 100 g of ethanol solution.
Molality is defined as the moles of the solute present in 1 kilogram of the solvent.
Given that:
Mass of
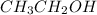 = 2 g
= 2 g
Molar mass of
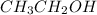 = 46.07 g/mol
= 46.07 g/mol
The formula for the calculation of moles is shown below:

Thus,
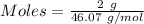
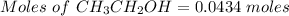
Mass of water = 100 - 2 g = 98 g = 0.098 kg ( 1 g = 0.001 kg )
So, molality is:
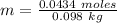
Molality = 0.4429 m