Answer : The mass of combustion products formed are 134 lbs.
Explanation :
The balanced chemical reaction will be:
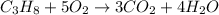
Given :
Mass of
 = 29 lbs = 13154.2 g
= 29 lbs = 13154.2 g
conversion used : 1 lbs = 453.592 g
Molar mass of
 = 44 g/mole
= 44 g/mole
First we have to calculate the moles of
 .
.
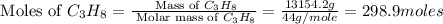
Now we have to calculate the moles of
 and
and
 .
.
From the balanced chemical reaction we conclude that,
As, 1 mole of
 react to give 3 moles of
react to give 3 moles of

So, 298.9 mole of
 react to give
react to give
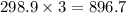 moles of
moles of

and,
As, 1 mole of
 react to give 4 moles of
react to give 4 moles of

So, 298.9 mole of
 react to give
react to give
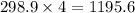 moles of
moles of

Now we have to calculate the mass of
 and
and
 .
.
Molar mass of
 = 44 g/mole
= 44 g/mole
Molar mass of
 = 18 g/mole
= 18 g/mole
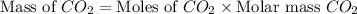
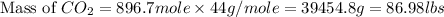
and,
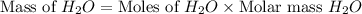
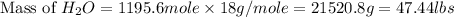
The total mass of products = Mass of
 + Mass of
+ Mass of

The total mass of products = 86.98 + 47.44 = 134.42 ≈ 134 lbs
Therefore, the mass of combustion products formed are 134 lbs.