Answer:Decreases
Step-by-step explanation:
Given
Volume is held constant that is it is a isochoric process.
We know that
PV=nRT
as n,V& R are constant therefore only variables are
P & T
so
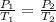
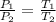
As
 is decreasing therefore Pressure must also decrease so that ratio remains constant.
is decreasing therefore Pressure must also decrease so that ratio remains constant.