Answer:
water
Step-by-step explanation:
The specific heat of an object denotes the amount of energy which is required to raise the objects temperature by 1 unit of temperature and mass of the object is 1 unit.
If the same amount of heat is added to both water and iron the temperature difference in their final and initial temperatures will be different.
The difference in the initial and final temperature of iron will be higher than that of water. This means that the amount of energy which is required to raise the objects temperature by 1 unit of temperature for iron is lower than water.
So, water has higher specific heat
Also
Q = mcΔT
where
Q = Heat
m = Mass
c = Specific heat
ΔT = Change in temperature
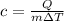
This means specific heat is inversely proportional to change in temperature.
So, for water the change in temperature will be lower than iron which means that water will have a higher specific heat.