Answer:
The pressure is 37.56 atm.
Temperature at the end of adiabatic compression is 825.73 K.
Step-by-step explanation:
Given that
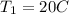
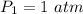
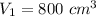
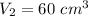
So r=800/60=13.33
γ=1.4
For process 1-2
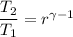
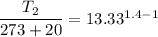
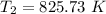
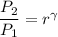
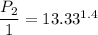
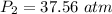
So
The pressure is 37.56 atm.
Temperature at the end of adiabatic compression is 825.73 K.