Answer:
K.E. = 5.4362 × 10⁻¹⁹ J
Step-by-step explanation:
The expression for Bohr velocity is:
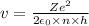
Applying values for hydrogen atom,
Z = 1
Mass of the electron (
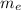 ) is 9.1093×10⁻³¹ kg
) is 9.1093×10⁻³¹ kg
Charge of electron (e) is 1.60217662 × 10⁻¹⁹ C
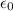 = 8.854×10⁻¹² C² N⁻¹ m⁻²
= 8.854×10⁻¹² C² N⁻¹ m⁻²
h is Plank's constant having value = 6.626×10⁻³⁴ m² kg / s
We get that:
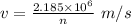
Given, n = 2
So,
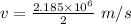
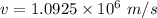
Kinetic energy is:

So,

K.E. = 5.4362 × 10⁻¹⁹ J