Answer:
4.186 × 10⁷ J
Step-by-step explanation:
Heat gain by water = Q
Thus,
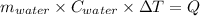
For water:
Volume = 1 m³ = 1000 L ( as 1 m³ = 1000 L)
Density of water= 1 kg/L
So, mass of the water:
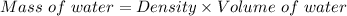
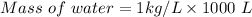
Mass of water = 1000 kg
Specific heat of water = 4.186 kJ/kg K
ΔT = 10 K
So,
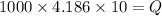
Q = 41860 kJ
Also, 1 kJ = 1000 J
So, Q = 4.186 × 10⁷ J