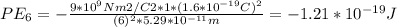Answer:
Potential energy for n = 6 Bohr orbit electron is -1.21*10⁻¹⁹J
Step-by-step explanation:
As per the Bohr model, the potential energy of electron in an nth orbit is given as:
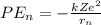
here:
k = Coulomb's constant = 9*10⁹ Nm2/C2
Z = nuclear charge
e = electron charge = 1.6*10⁻¹⁹ C
r(n) = radius of the nth orbit = n²(5.29*10⁻¹¹m)
Substituting for k, Z(= 1), e and r(n) in the above equation gives: