Answer:
change in enthalpy for 1 lb water is - 970.10872 Btu
Step-by-step explanation:
Given data:
weight of water 1 lb
pressure 1 atm
temperature 212 Fahrenheit
From steam table
initial enthalpy
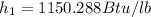
final enthalpy
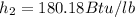
change in enthalpy is given as
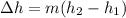
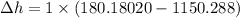
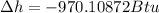
change in enthalpy for 1 lb water is - 970.10872 Btu