Step-by-step explanation:
Boiling point is defined as the point at which liquid state and vapor state of a substance are existing in equilibrium.
Equilibrium is defined as the state in which rate of forward and rate of backward reaction are equal to each other.
For example,
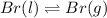
So, when we boil bromine which is present in liquid state then at the boiling point its vapors will exist in equilibrium. And unless all the liquid state of bromine will not convert into vapors its temperature will not change.
Therefore, we can conclude that at boiling point the liquid and the vapur of Bromine are in equilibrium.