Answer: The cell potential of the cell is 0.31 V
Step-by-step explanation:
We know that:
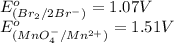
The substance having highest positive
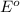 potential will always get reduced and will undergo reduction reaction. Here,
potential will always get reduced and will undergo reduction reaction. Here,
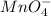 will undergo reduction reaction will get reduced. And, bromine will get oxidized.
will undergo reduction reaction will get reduced. And, bromine will get oxidized.
Oxidation half reaction:
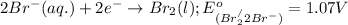 ( × 5)
( × 5)
Reduction half reaction:
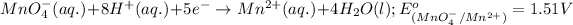 ( × 2)
( × 2)
The net reaction follows:
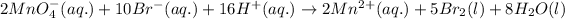
Oxidation reaction occurs at anode and reduction reaction occurs at cathode.
To calculate the
 of the reaction, we use the equation:
of the reaction, we use the equation:
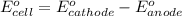
Putting values in above equation, we get:
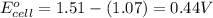
To calculate the EMF of the cell, we use the Nernst equation, which is:
![E_(cell)=E^o_(cell)-(0.059)/(n)\log ([Mn^(2+)]^2)/([MnO_4^(-)]^2* [Br^-]^(10)* [H^+]^(16))](https://img.qammunity.org/2020/formulas/chemistry/college/2ltnxsvcz48fm0xdn83p35323ndmwk8005.png)
where,
 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V
 = standard electrode potential of the cell = 0.44 V
= standard electrode potential of the cell = 0.44 V
n = number of electrons exchanged = 10
![[H^(+)]=1M](https://img.qammunity.org/2020/formulas/chemistry/college/beznoijlurrflsj0ibtg83pej05ssbxxka.png)
![[Mn^(2+)]=0.15M](https://img.qammunity.org/2020/formulas/chemistry/college/ulblbc513kifoxgnobsp3ujktxy24hh0oh.png)
![[MnO_4^(-)]=0.01M](https://img.qammunity.org/2020/formulas/chemistry/college/sxttcr6pg5sqyzhmkznmh6jb03qsu71r2d.png)
![[Br^(-)]=0.01M](https://img.qammunity.org/2020/formulas/chemistry/college/73whcn88fli6mekmpba524e07zegekpxbs.png)
Putting values in above equation, we get:
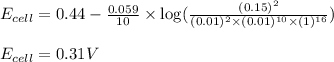
Hence, the cell potential of the cell is 0.31 V