Answer:
Heat required =7126.58 Btu.
Step-by-step explanation:
Given that
Mass m=20 lb
We know that
1 lb =0.45 kg
So 20 lb=9 kg
m=9 kg
Ice at -15° F and we have to covert it at 200° F.
First ice will take sensible heat at up to 32 F then it will take latent heat at constant temperature and temperature will remain 32 F.After that it will convert in water and water will take sensible heat and reach at 200 F.
We know that
Specific heat for ice
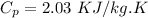
Latent heat for ice H=336 KJ/kg
Specific heat for ice
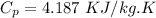
We know that sensible heat given as
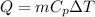
Heat for -15F to 32 F:
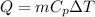
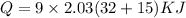
Q=858.69 KJ
Heat for 32 Fto 200 F:
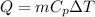
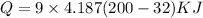
Q=6330.74 KJ
Total heat=858.69 + 336 +6330.74 KJ
Total heat=7525.43 KJ
We know that 1 KJ=0.947 Btu
So 7525.43 KJ=7126.58 Btu
So heat required to covert ice into water is 7126.58 Btu.