Answer:
Total feed rate = 98.3 lbmol/h
methanol mole fraction = 0.315
water mole fraction = 0.685
Step-by-step explanation:
First of all, it is needed to calculate the feed mass of methanol and water in kg/h.
For methanol:
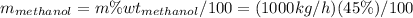
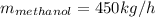
For water:
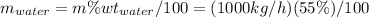
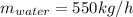
Now, change from mass units (kg/h) to moles units (kmol/h and lbmol/h) using simple conversion factors:
For methanol:
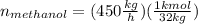
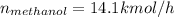
For water:
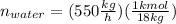
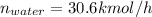
Change units from kmol/h to lbmol/h
For methanol:
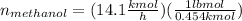
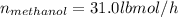
For water:
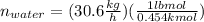
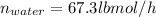
Sum moles of methanol and water in lbmol/h to compute the total feed rate:
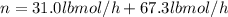
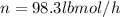
Divide both methanol and water moles feed rates by total feed rate:
For methanol:
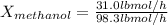
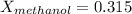
For water:
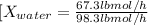
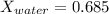
End