Step-by-step explanation:
For an isothermal process equation will be as follows.
W = nRT ln

It is given that mass is 10 kg/s or 10,000 g/s (as 1 kg = 1000 g). So, calculate number of moles of water as follows.
No. of moles =

=
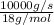
= 555.55 mol/s
= 556 mol/s (approx)
As T =
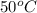 or (50 + 273.15) K = 323.15 K. Hence, putting the given values into the above formula as follows.
or (50 + 273.15) K = 323.15 K. Hence, putting the given values into the above formula as follows.
W = nRT ln[/tex]\frac{P_{1}}{P_{2}}[/tex]
=
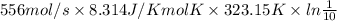
=
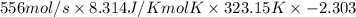
= -3440193.809 J/s
Negative sign shows work is done by the pump. Since, 1 J = 0.001 kJ. Therefore, converting the calculated value into kJ as follows.
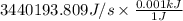
= 3440.193 kJ/s
= 3451 kJ/s (approx)
Thus, we can conclude that the pump work is 3451 kJ/s.