Answer: Option (a) is the correct answer.
Step-by-step explanation:
The given data is as follows.
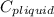 = 4.19
= 4.19
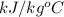
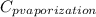 = 1.9
= 1.9
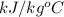
Heat of vaporization (
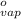 ) at 1 atm and
) at 1 atm and
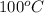 is 2259 kJ/kg
is 2259 kJ/kg
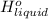 = 0
= 0
Therefore, calculate the enthalpy of water vapor at 1 atm and
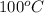 as follows.
as follows.
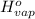 =
=
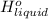 +
+
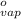
= 0 + 2259 kJ/kg
= 2259 kJ/kg
As the desired temperature is given
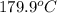 and effect of pressure is not considered. Hence, enthalpy of liquid water at 10 bar and
and effect of pressure is not considered. Hence, enthalpy of liquid water at 10 bar and
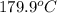 is calculated as follows.
is calculated as follows.
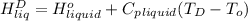
=
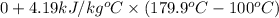
= 334.781 kJ/kg
Hence, enthalpy of water vapor at 10 bar and
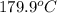 is calculated as follows.
is calculated as follows.
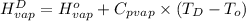
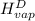 =
=
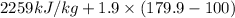
= 2410.81 kJ/kg
Therefore, calculate the latent heat of vaporization at 10 bar and
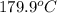 as follows.
as follows.
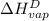 =
=
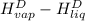
= 2410.81 kJ/kg - 334.781 kJ/kg
= 2076.029 kJ/kg
or, = 2076 kJ/kg
Thus, we can conclude that at 10 bar and
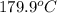 latent heat of vaporization is 2076 kJ/kg.
latent heat of vaporization is 2076 kJ/kg.