Answer:
Vinegar has 4.09% of acetic acid.
Step-by-step explanation:
The neutralization reaction is:
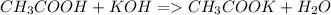
Each mol of KOH reacts with each mole of acetic acid so the quantity of moles of acetic acid is:
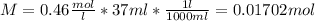
The mass of acetic acid is:
0.01702 mol×60.02g/mol=1.0215 g Acetic Acid
Finally, the percentage is:
%=1.0215 g Acetic Acid÷25g vinegar(solution)=4.09%