Step-by-step explanation:
Isotope are the species which have same atomic number (number of the protons) but number of neutrons different. which corresponds to different mass of isotopes.
For example,
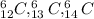 are the three isotopes of carbon.
are the three isotopes of carbon.
Isoelectronic are the species which have the same number of electrons.
For example,
Ne is isoelectronic with
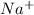
Isotopes of
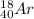 are:
are:
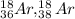
The number of the electrons in
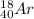 = 18
= 18
Number of the electrons in
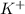 = 18
= 18
Thus,
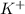 is isoelectronic to Argon-40
is isoelectronic to Argon-40