Answer:
C)185,500 KJ
Step-by-step explanation:
Given that
Latent heat fusion = 333.23 KJ/kg
Latent heat vaporisation = 333.23 KJ/kg
Mass of ice = 100 kg
Mass of water = 40 kg
Mass of vapor=60 kg
Ice at 0°C ,first it will take latent heat of vaporisation and remain at constant temperature 0°C and it will convert in to water.After this water which at 0°C will take sensible heat and gets heat up to 100°C.After that at 100°C vapor will take heat as heat of vaporisation .
Sensible heat for water Q
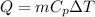
For water
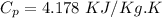
Q=4.178 x 40 x 100 KJ
Q=16,712 KJ
So total heat
Total heat =100 x 333.23+16,712 + 60 x 2257 KJ
Total heat =185,455 KJ
Approx Total heat = 185,500 KJ
So the answer C is correct.