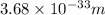Answer : The de Broglie wavelength will be
Solution :
The formula used for de Broglie wavelength is:
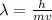 ..........(1)
..........(1)
where,
 = wavelength = ?
= wavelength = ?
h = Planck's constant =
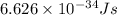
m = mass = 1.2 g = 0.0012 kg
Conversion used : 1 kg = 1000 g
v = velocity = 150 m/s
Now put all the given values in equation 1, we get:
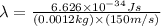
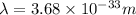
Therefore, the de Broglie wavelength will be