Answer : The change in molar entropy of the sample is 10.651 J/K.mol
Explanation :
To calculate the change in molar entropy we use the formula:
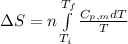
where,
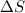 = change in molar entropy
= change in molar entropy
n = number of moles = 1.0 mol
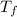 = final temperature = 300 K
= final temperature = 300 K
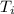 = initial temperature = 273 K
= initial temperature = 273 K
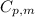 = heat capacity of chloroform =
= heat capacity of chloroform =
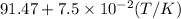
Now put all the given values in the above formula, we get:
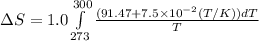
![\Delta S=1.0* [91.47\ln T+7.5* 10^(-2)T]^(300)_(273)](https://img.qammunity.org/2020/formulas/chemistry/college/6hq70gj4f61xd1is5ercvd8f6xo19qevso.png)
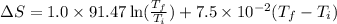
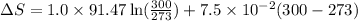
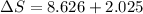
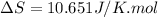
Therefore, the change in molar entropy of the sample is 10.651 J/K.mol