Answer:
1.930 * 10⁻⁹ mg of Mn⁺² are left unprecipitated.
Step-by-step explanation:
The reaction that takes place is:
Mn⁺² + S⁻² ⇄ MnS(s)
ksp = [Mn⁺²] [S⁻²]
If the pksp of MnS is 13.500, then the ksp is:
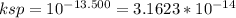
From the problem we know that [S⁻²] = 0.0900 M
We use the ksp to calculate [Mn⁺²]:
3.1623*10⁻¹⁴= [Mn⁺²] * 0.0900 M
[Mn⁺²] = 3.514 * 10⁻¹³ M.
Now we can calculate the mass of Mn⁺², using the volume, concentration and atomic weight. Thus the mass of Mn⁺² left unprecipitated is:
3.514 * 10⁻¹³ M * 0.1 L * 54.94 g/mol = 1.930 * 10⁻¹² g = 1.930 * 10⁻⁹ mg.