Answer:
100Btu
Step-by-step explanation:
According to the First law of thermodynamics:
ΔQ = W + ΔU
Where,
ΔQ is the heat change
W is the amount of work done
ΔU is change in internal energy
Since, there is no work done on the system as heat is just passing from coffee mug to surroundings, So, W = 0
Thus,
Net heat change = change in internal energy.
Change in internal energy =
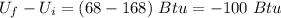
Thus,
ΔQ = -100 Btu (negative sign indicates release of heat)
So,
Heat flown to the room = 100Btu