Answer: The molar mass of the unknown gas is 18.3 g/mol
Step-by-step explanation:
To calculate the rate of diffusion of gas, we use Graham's Law.
This law states that the rate of effusion or diffusion of gas is inversely proportional to the square root of the molar mass of the gas. The equation given by this law follows the equation:
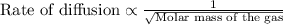
We are given:
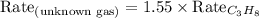
We know that:
Molar mass of propane = 44 g/mol
Taking the ratio of the rate of effusion of the gases, we get:
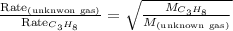
Putting values in above equation, we get:
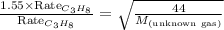

Hence, the molar mass of the unknown gas is 18.3 g/mol