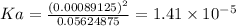Answer:
Ka of nicotinic acid =
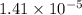
Step-by-step explanation:
pH = 3.05
![pH = -log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/college/ubz795vlg6nmsmnx3ybpt5q3hj348r3i8k.png)
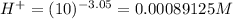
No. of mol of nicotinic acid =
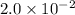
Volume of water = 350 mL = 0.0350 L
Molarity =
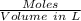
Molarity =
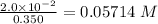
Nicotinic acid dissoctates as:
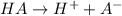
[H+] = 0.00089125 M
[A-] = 0.00089125 M
[HA} at equilibium = 0.05714 - 0.00089125 = 0.05624875 M
![Ka = ([H^+][A^-])/([HA])](https://img.qammunity.org/2020/formulas/chemistry/college/9pq20t1x34u5yiad1qvrcf6gp3oilijnbr.png)