Answer: The correct answer is Option d.
Step-by-step explanation:
We are given:
Mass percentage of
 = 20 %
= 20 %
So, mole fraction of
 = 0.2
= 0.2
Mass percentage of
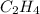 = 30 %
= 30 %
So, mole fraction of
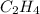 = 0.3
= 0.3
Mass percentage of
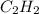 = 35 %
= 35 %
So, mole fraction of
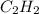 = 0.35
= 0.35
Mass percentage of
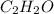 = 15 %
= 15 %
So, mole fraction of
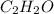 = 0.15
= 0.15
We know that:
Molar mass of
 = 16 g/mol
= 16 g/mol
Molar mass of
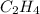 = 28 g/mol
= 28 g/mol
Molar mass of
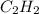 = 26 g/mol
= 26 g/mol
Molar mass of
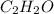 = 48 g/mol
= 48 g/mol
To calculate the average molecular mass of the mixture, we use the equation:
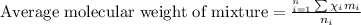
where,
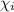 = mole fractions of i-th species
= mole fractions of i-th species
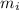 = molar masses of i-th species
= molar masses of i-th species
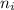 = number of observations
= number of observations
Putting values in above equation:
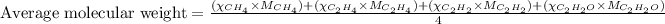
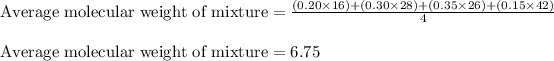
Hence, the correct answer is Option d.