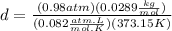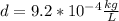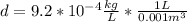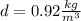Answer:
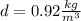
Step-by-step explanation:
Using the Ideal Gas Law we have
 and the number of moles n could be expressed as
and the number of moles n could be expressed as
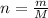 , where m is the mass and M is the molar mass.
, where m is the mass and M is the molar mass.
Now, replacing the number of moles in the equation for the ideal gass law:
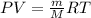
If we pass the V to divide:
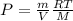
As the density is expressed as
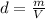 , we have:
, we have:
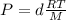
Solving for the density:
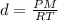
Then we need to convert the units to the S.I.:
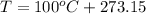
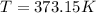
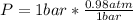
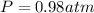
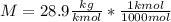
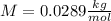
Finally we replace the values: