Answer:
X1= 41%
heat transfer = -3450.676 KJ
Step-by-step explanation:
To get the properties for pure substance in a system we need to know at least to properties. These are usually pressure and temperature because they’re easy to measure. In this case we know the initial pressure (20 bar) which is not enough to get all the properties, but they ask to determine quality, this a property that just have meaning in the two-phase region (equilibrium) so with this information we can get the temperature of the system and all its properties.
There is another property that we can calculate from the data. This is the specific volume. This is defined as
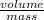 . We know the mass (12 Kg) and we can assume the volume is the volume of the tank (0.5
. We know the mass (12 Kg) and we can assume the volume is the volume of the tank (0.5
 ) because they say that the tank was filled.
) because they say that the tank was filled.
With this we get a specific volume of
Specific volume =
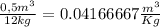
From the thermodynamic tables we can get the data for the saturated region with a pressure of 20 bar.
Temperature of saturation = 212.385 °C
Specific volume for the saturated steam (vg) = 0.0995805
![\frac{m^(3)}/{Kg}[\tex]</p><p></p><p>Specific volume for the saturated liquid (vf)= 0.00117675 [tex]\frac{m^(3)}/{Kg}[\tex]</p><p> </p><p>The specific volume that we calculate before 0.04166667 m^3/Kg is between 0.00117675 m^3/Kg and 0.0995805 m^3/Kg so we can be sure that we are in two-phase region (equilibrium).</p><p></p><p>The quality (X) is defined as the percentage in mass of saturated steam in a mix (Two-phase region)</p><p></p><p> The relation between specific volume and quality is </p><p>[tex]v = (1-x)*v_(f) + x*v_(g)[\tex] </p><p>where </p><p>v in the specific volume in the condition (0.04166667 m^3/Kg) </p><p>vf = Specific volume for the saturated liquid (0.00117675 m^3/Kg)</p><p></p><p>vg = Specific volume for the saturated steam (0.0995805 m^3/Kg)</p><p></p><p>x = quality</p><p></p><p>clearing the equation we get:</p><p></p><p>[tex]X = ((v-v_(f)))/((v_(g)-v_(f)))]()
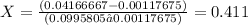
The quality is 41%
To calculate the heat transfer we use the next equation.
Q = m * Cp * delta T
Where
Q = heat transfer (Joules, J)
m= mass of the substance (g)
Cp = specific heat (J/g*K) from tables
Delta T = change in temperature in K for this equation.
The mass of the substance is 12 kg or 12000 g for this equation
Cp from tables is 4,1813 J/g*K. You can find this value for water in different states. Here we are using the value for liquid water.
For delta T, we know the initial temperature 212.385 °C.
We also know that the system was cooled. Since we don’t have more information, we can assume that the system was cooled until a condition where all the steam condensates so now we have a saturated liquid. Since we know the pressure (4 bar), we can get the temperature of saturation for this condition from the thermodynamics tables. This is 143.613 °C, so this is the final temperature for the system.
T(K) = T°C +273
T1(K) = 212.385 + 273.15 = 485.535 K
T2 (K) = 143.613 +273.15= 416.763 K
Delta T (K) = (T2-T1) =416.763 K - 485.535 K = -68.772 K
Now we can calculate Q
Q = 12000g * 4,1813 J/g*K* (-68.772 K) = -3450676.36 J or -3450.676 KJ
Is negative because the heat is transfer from the water to the surroundings