Answer:
The rate constant of the reaction is
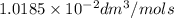 .
.
 is the half life of the reactant.
is the half life of the reactant.
Step-by-step explanation:
A+B → P
Integrated rate law for second order kinetics is given by:
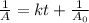
A = concentration left after time t =
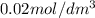
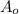 = Initial concentration =
= Initial concentration =
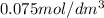
t = 1 hour = 3600 seconds
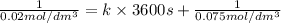
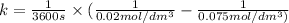
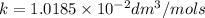
Half life for second order kinetics is given by:
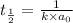
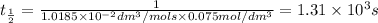
The rate constant of the reaction is
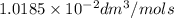 .
.
 is the half life of the reactant.
is the half life of the reactant.