Answer:
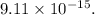
Step-by-step explanation:
The water droplet is initially neutral, it will obtain a 40 nC of charge when a charge of -40 nC is removed from the water droplet.
The charge on one electron,
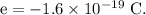
Let the N number of electrons have charge -40 nC, such that,
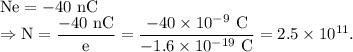
Now, mass of one electron =
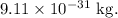
Therefore, mass of N electrons =
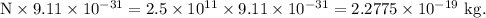
It is the mass of the of the water droplet that must be removed in order to obtain a charge of 40 nC.
Let it is m times the total mass of the droplet which is
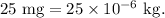
Then,
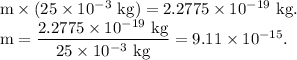
It is the required fraction of mass of the droplet.