Answer: Work done for the process is -390 J
Step-by-step explanation:
The chemical equation for the reaction of zinc metal with sulfuric acid follows:
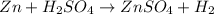
To calculate the number of moles, we use the equation:
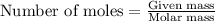
Given mass of zinc = 10.0 g
Molar mass of zinc = 65.38 g/mol
Putting values in above equation, we get:
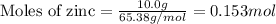
The equation given by ideal gas follows:
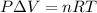
where, P = pressure of the gas
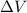 = Change in volume of the gas
= Change in volume of the gas
T = Temperature of the gas = 298 K
R = Gas constant = 8.314 J/mol.K
n = number of moles of gas = 0.153 mol
Putting values in above equation, we get:
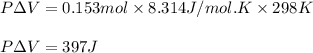
To calculate the work done, we use the equation:
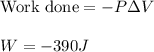
Hence, work done for the process is -390 J