Answer : The mass of sodium sulfate needed is 5.7085 grams.
Explanation : Given,
Concentration of sodium ion = 0.148 mol/L
Volume of solution = 2.29 L
Molar mass of sodium sulfate = 142 g/mole
First we have to determine the moles of sodium ion.
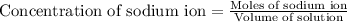
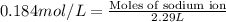
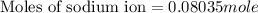
Now we have to calculate the moles of sodium sulfate.
The balanced chemical reaction will be,
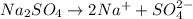
As, 2 moles of sodium ion produced from 1 moles of
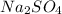
So, 0.08035 moles of sodium ion produced from
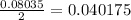 moles of
moles of
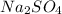
Now we have to calculate the mass of sodium sulfate.
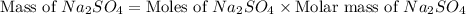
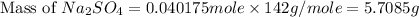
Therefore, the mass of sodium sulfate needed is 5.7085 grams.