Answer:
18 kJ
Step-by-step explanation:
Given:
Initial volume of air = 0.05 m³
Initial pressure = 60 kPa
Final volume = 0.2 m³
Final pressure = 180 kPa
Now,
the Work done by air will be calculated as:
Work Done = Average pressure × Change in volume
thus,
Average pressure =
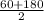 = 120 kPa
= 120 kPa
and,
Change in volume = Final volume - Initial Volume = 0.2 - 0.05 = 0.15 m³
Therefore,
the work done = 120 × 0.15 = 18 kJ