Answer:
The amount of drug required = 44.44 mL
Diluent needed = 355.56 mL
Explanation:
Data provided in the question:
Total volume of solution = 400 mL
Concentration of drug = 1 : 8
Now,
The ratio is interpreted as 1 part of drug and 8 part of diluent
Thus,
The amount of drug required =
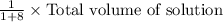
or
The amount of drug required =
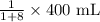
or
The amount of drug required = 44.44 mL
and,
Diluent needed =
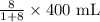
or
Diluent needed = 355.56 mL