Answer:
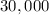 mL
mL
Step-by-step explanation:
Given -
Volume of buffer
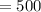 mL
mL
Concentration of solution
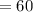 mM
mM
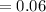 M
M
New concentration of the solution
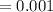 M
M
As we know
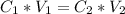
Where
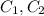 are the concentration of the solution before and after dilution.
are the concentration of the solution before and after dilution.
And
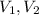 are the volume of solution before and after dilution.
are the volume of solution before and after dilution.
Substituting the given values in above equation, we get -
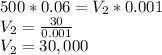 mL
mL