Answer:
The measured weight of the water = 4.93 g
The percentage error in measured value = 1.4%
Explanation:
Given:
Weight of the 10-mL graduate = 42.745 g
Combined weight of graduate and 5 mL water = 47.675 g
5 mL of water should weight = 5 g
thus, actual value of 5 mL water = 5 g
Now,
The measured weight of the water
= (Combined weight of graduate + 5 mL water) - Weight of the 10-mL graduate
= 47.675 g - 42.745 g
= 4.93 g
The percentage error in measured value
=
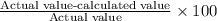
=
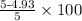
or
= 1.4%