Answer:
1.2 grams.
Explanation:
We have been given that an oral concentrate of sertraline hydro-chloride (Zoloft) contains 20 mg/mL of the drug.
First of all, we will find number of mg in 60 mL container of the concentrate as:
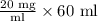
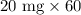
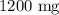
We know 1 gram equals 1000 mg.
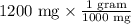
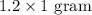
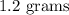
Therefore, 1.2 grams of sertraline hydrochloride are in each 60-mL container of the concentrate.