Answer:
5mL would be required to provide a patient with 0.25 mg of the drug substance.
Explanation:
The problem states that an injectable solution contains 25μg of a dug substance in each 0.5 mL, and asks how many milliliters would be required to provide a patient with 0.25 mg of the drug substance.
So, the first step is the conversion of 25ug to mg, since the problem asks the answer in mg.
Each mg has 1000ug. So
1mg - 1000ug
xmg - 25ug
1000x = 25
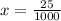
x = 0.025 mg
It means that each 0.5mL of the solution contains 0.025mg of the drug. How many milliliters would be required to provide a patient with 0.25 mg of the drug substance.
0.5mL - 0.025mg
xmL - 0.25mg
0.025x = 0.5*0.25
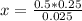
x = 10*0.5
x = 5mL
5mL would be required to provide a patient with 0.25 mg of the drug substance.