Answer:
Na
Step-by-step explanation:
To find the limiting reactant we must first write the balanced chemical reaction. It must be correctly balanced so that we can find the proper mole ratios.
2 Na + H₂ → 2 NaH
After this we will convert our measurements to moles. For mass we do this by dividing by the molar mass.
6.75g ÷ 22.99 = 0.294mol Na
3.03g ÷ 2.02 = 1.50mol H₂
Now that we have the moles of each of the reactants, we can multiply them by their mole ratio with a reactant.
0.294mol Na ×
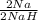 = 0.294mol NaH
= 0.294mol NaH
1.50mol H₂ × 2/1 = 3.00mol NaH
Na is our limiting reagent because it makes the smaller amount of moles.