Answer:
The mass of Fe₂O₃ in 0.500 g of mixture is 0.367 g.
Step-by-step explanation:
First off, we know that 72% of the mass of the mixture is iron. The information also tells us that the remaining 28% of the mass is oxygen.
Now we calculate the total mass of iron and the total mass of oxygen in the mixture:
- 0.500 g * 0.72 = 0.360 g of Fe
- 0.500 g * 0.28 = 0.140 g of O
With the mass of each element we can calculate the number of moles of each atom, using the atomic weight:
0.360 g Fe * 1 mol / 55.845 g = 0.00645 moles of Fe
0.140 g O * 1 mol / 16 g = 0.00875 moles of O
The number of moles of Fe in the mixture is equal to the number of moles of FeO plus two times the number of moles of Fe₂O₃:
0.00645 =
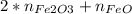 eq A
eq A
The number of moles of O in the mixture is equal to the number of moles of FeO plus three times the number of moles of Fe₂O₃:
0.00875 =
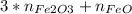 eq B
eq B
So now we have a system of two equations and two unknowns, we solve for
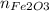 :
:
From eq A:
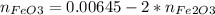
Replacing in eq B:
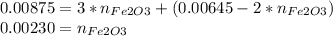
Now we just need to convert moles of Fe₂O₃ into grams, using the molecular weight:
0.00230 moles * 159.66 g/mol = 0.367 g Fe₂O₃