Answer:
5.619 grams of diphosphorus pentoxide are formed when 2.45 g of phosphorus reacts with excess oxygen.
Step-by-step explanation:
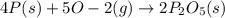
Mass of phosphorus = 2.45 g
Moles of phosphorous =
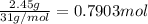
According to reaction 4 moles of phosphorus gives 2 moles of diphosphorus pentoxide.
Then 0.7903 moles of phosphorus will give:
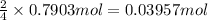 of diphosphorus pentoxide
of diphosphorus pentoxide
Mass of 0.03957 moles of diphosphorus pentoxide :
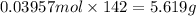
5.619 grams of diphosphorus pentoxide are formed when 2.45 g of phosphorus reacts with excess oxygen.