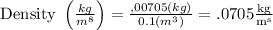A 10.0cm3 volume of alcohol has a mass of 7.05g.
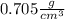 is the density
is the density
Answer:
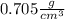
Step-by-step explanation:
The ratio between the mass of a substance and its unit volume is referred as density.
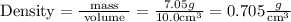 is the Answer
is the Answer
Note:
1000 g = 1 kg
100 cm = 1 m
When we used to transform the given values into S.I units, then
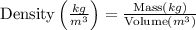
Alcohol’s volume can be written as,
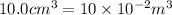
Alcohol’s mass can denote as,
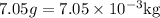
Then,