Answer:
33.4°C .
Step-by-step explanation:
mass of bullet, m = 15 g = 0.015 kg
velocity of bullet, v = 1502 m/s
mass of wax, M = 2.5 kg
Initial temperature of wax, T1 = 31°C
Let T2 be the final temperature of wax.
Specific heat of wax, c = 0.7 cal/g°C = 0.7 x 1000 x 4 J/kg°C = 2800 J/kg°C
The kinetic energy of the bullet is converted into heat energy which is used to heat the wax.
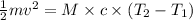
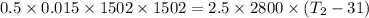
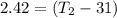
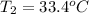
thus, the final temperature of wax is 33.4°C .