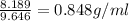Answer:
density of liquid 0.848 g/ml
Step-by-step explanation:
from the information given in the question
mass of water = 34.914 - 25.296 = 9.618 g
volume of pycnometer = volume of water
which will be equal to
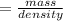
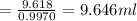
mass of liquid =33.485-25.296 = 8.189 ml
density of liquid
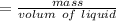
=