Answer:
B) Na has a lower first ionization energy than Ne.
Step-by-step explanation:
The atomic number¹ for Na has a value of 11 while in the case of Ne this value is 10. That means that Sodium (Na) has a total number of 11 protons, 11 neutrons and 11 electrons (since it is electrically neutral²). For the case of Neon (Ne) it has 10 protons, 10 neutrons and 10 electrons.
As the atomic number increases, the atomic radius³ shrinks (the orbitals are closer to the nucleus) as a consequence of the electric force. For the case of sodium (Na) the electron in the outermost orbital will experience a lower electric force than the electron placed in the outermost orbital in the atom of Neon (Ne).
Although, the sodium’s atom has more protons and therefore electrons, these eleven electrons will be organized according with the electronic configuration⁴ in the different shells (orbitals) of probabilities of their positions around the atom.
The electronic configuration for Na is:
1s²2s²2p⁶3s¹
The electronic configuration for Ne is:
1s²2s²2p⁶
Since Na needs another orbital to placed its outermost electron, the atomic radius will have a greater value than Ne. The electric force is inversely proportional to the square of the distance between two charged particles, as is established in Coulomb’s law:
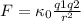 (1)
(1)
Where q1 and q2 are the charges,
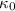 is the proportionality constant and r is the distance between the two charges.
is the proportionality constant and r is the distance between the two charges.
Hence, the electron in the outermost orbital of Ne is submitted to a greater electric force according with equation 1, the required energy to remove it (ionization energy⁵) will be greater than in the case of Na (for that case will be the first ionization energy).
¹Atomic number: The number of protons or electrons in an atom.
²Electricaly neutral: All the charges are balanced (same number of positive charges and negative charges).
³Atomic radius: Distance between the center of the nucleus and an electron placed in the outermost orbital for a specific atom.
⁴Electronic configuration: Show how the electrons of an atom will be arranged in different orbitals according with the fact that each orbital has a specific number of electrons that can be held.
⁵Ionization energy: Energy required to remove an electron from an atom.
Key values:
First ionization energy of Na: 495 kJ/mol
First ionization energy of Ne: 2080 kJ/mol
Atomic radius of Na: 2.27 Å
Atomic radius of Ne: 1.54 Å
Atomic number of Na: 11
Atomic number of Ne: 10